
SimplerQMS
Stay compliant and work smarter with SimplerQMS!
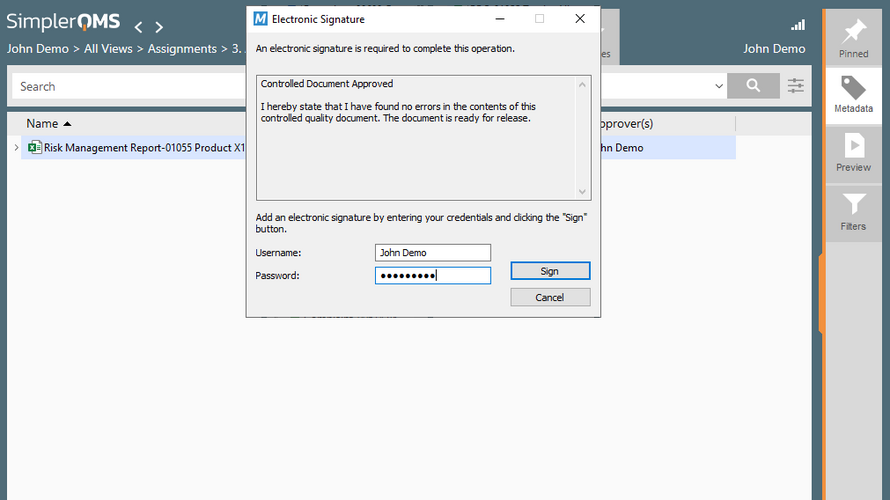
SimplerQMS is an ISO 13485:2016 certified, ready-to-use Quality Management Software designed specifically for life science organizations. With our pre-validated, cloud-based solution, we enable your company to work efficiently and paperless, while being in compliance with the regulatory authorities. All relevant life science modules – SOPs, Training, Complaints, CAPAs, Audit, Technical Documentation Management, and more, are integrated into one QMS Software solution. SimplerQMS ensures compliance by providing secure, FDA 21 CFR Part 11 compliant electronic signatures, time-stamped audit trails, and reporting capabilities. SimplerQMS helps you assure continuous compliance with FDA 21 CFR Part 11, ISO 13485, 21 CFR Part 820, GAMP5, EU MDR, EU IVDR, and other regulations.
Licensing & Deployment
-
Cloud Hosted
-
Web-based
-
iPhone/iPad
-
Android
-
Windows
-
Mac
Support
-
Phone
Knowledge Base
-
Help Guides
-
Video Guides
-
Blogs
Media
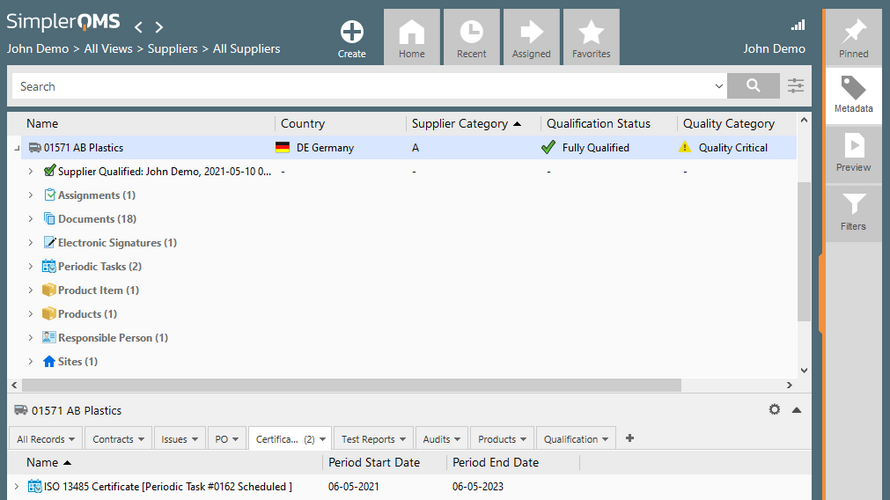
SimplerQMS Core Features
Focus of Quality Management Feature
- Audit Management
- Complaint Management
- Defect Tracking
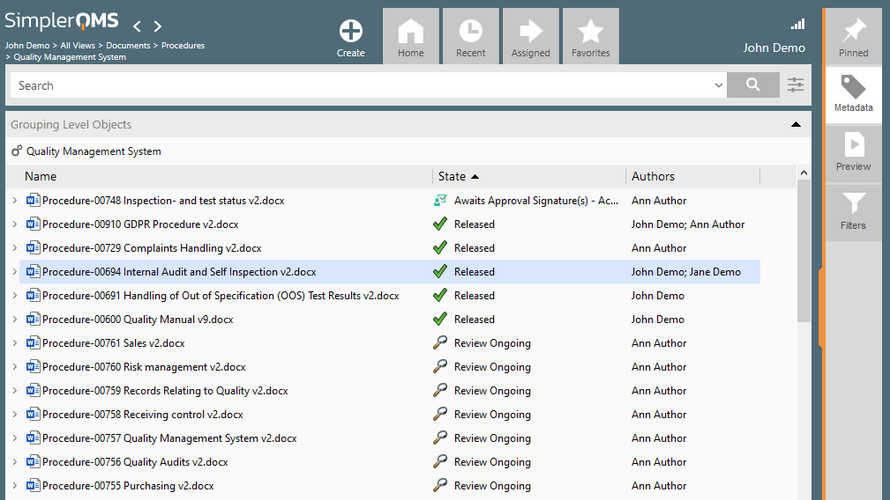
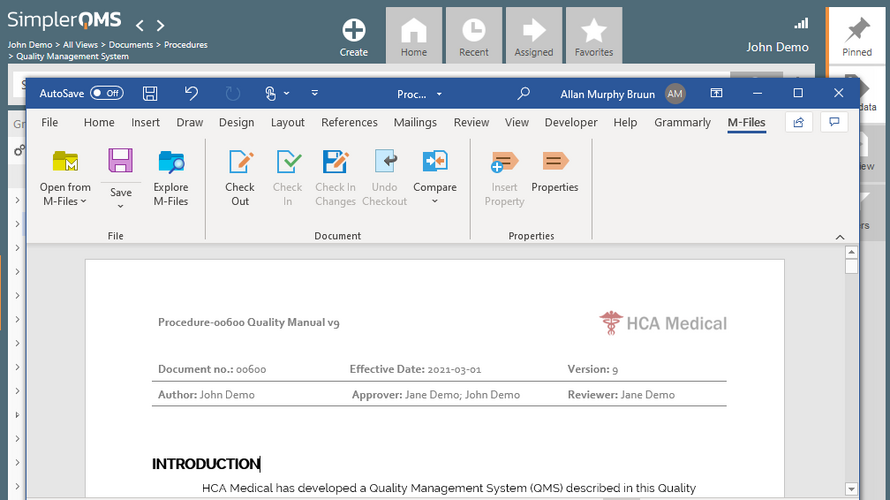
- Document Control
- Equipment Management
- Maintenance Management
- Performance Reporting
- Risk Management
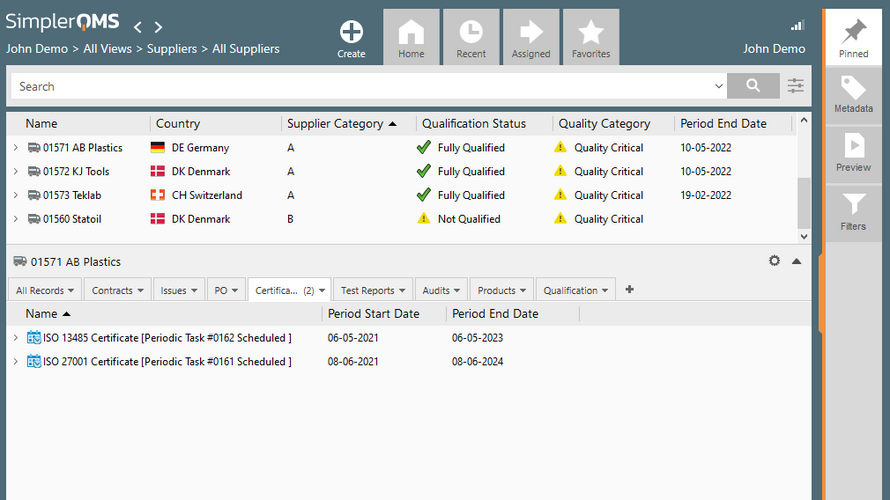
- Supplier Quality Control
- Training Management
SimplerQMS Pricing
Pricing Type
-
Contact Vendor
Free Version
-
No
Payment Frequency
-
Annual Subscription
-
Quote Based