
ClinicalPursuit
Rapid EDC Clinical Trial Data Management
ClinicalPURSUIT Electronic Data Management Software can perform electronic data capture (EDC) for dozens or thousands of patients. All company focused on clinical trial studies can benefit from this powerful EDC and Clinical Trail Data Management platform. These include sponsors, clinical research organizations (CROs), pharmaceuticals companies, biotech, and device manufacturers.
1998
United States
- Medical-practice
- English
Industries
-
Medical-practice
Licensing & Deployment
-
Proprietary
-
Cloud Hosted
-
Web-based
-
iPhone/iPad
-
Android
-
Windows
Support
-
Phone
-
24x7 Support
Media
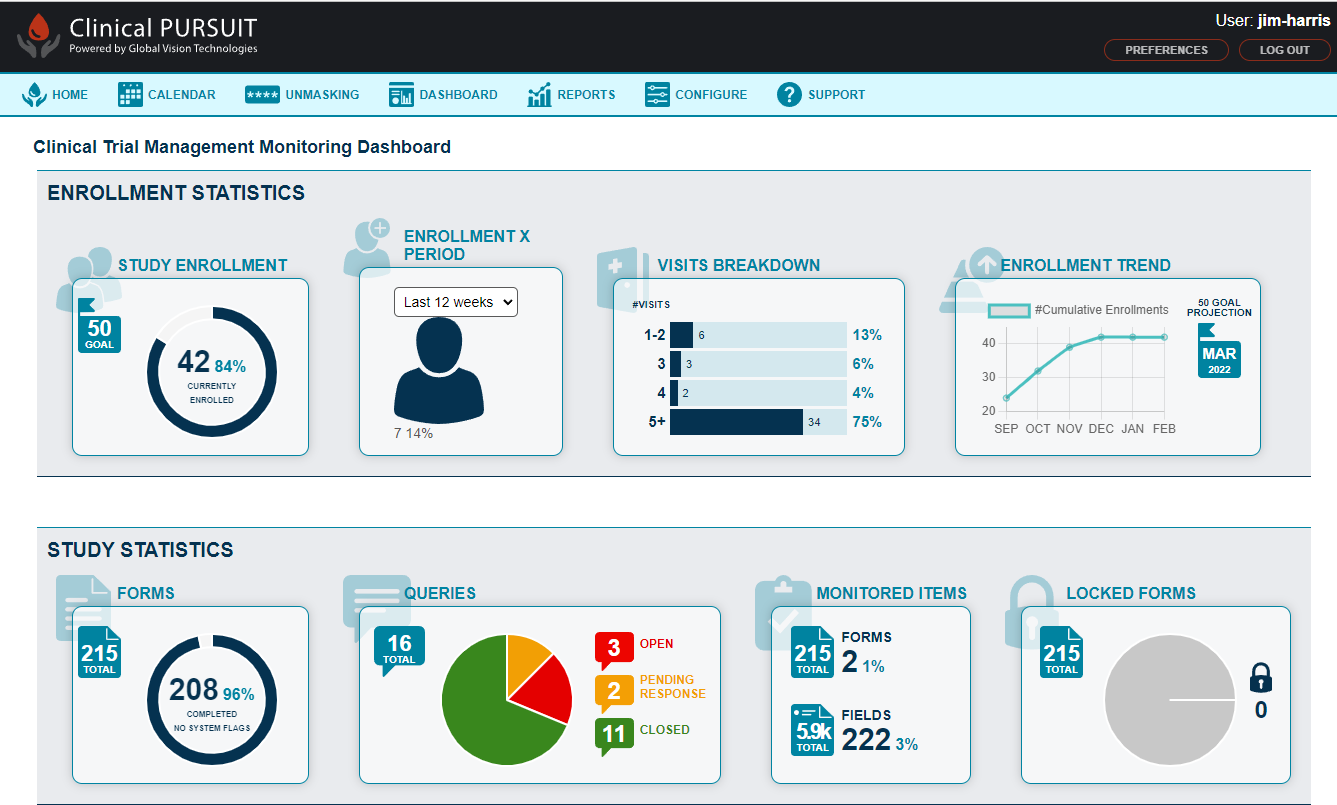
ClinicalPursuit Core Features
Focus of Clinical Trial Management Feature
- Compliance Management
- Document Management
- Electronic Data Capture
- Enrollment Management
- Financial Management
- HIPAA Compliant
- Monitoring
- Patient Database
- Payments Processing
- Recruiting Management
- Scheduling
- Study Planning
ClinicalPursuit Pricing
Pricing Type
-
Contact Vendor
Preferred Currency
-
USD ($)
Free Version
-
No
Payment Frequency
-
Annual Subscription